Kaufman Lab
- People
- Research & Publications
- Research Opportunities
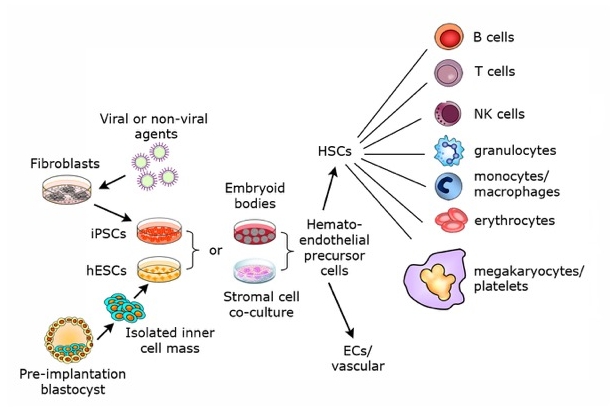
Figure from Kaufman, 2009
Studies in the Kaufman Lab focus on use of human pluripotent stem cells to study basic mechanisms that regulate early human blood cell development and to derive therapeutic mature blood cell populations.
These studies utilize both human embryonic stem cells (hESCs) and human induced pluripotent stem cells (iPSCs). Both hESCs and iPSCs can be maintained in long-term culture with stable karyotype and without loss of the developmental potential to make all the cells and tissues in the body. Therefore hESCs/iPSCs provide an ideal platform both for studies of human hematopoiesis and large-scale production of blood cells such as lymphocytes that can be used for novel therapies against cancer and other diseases.
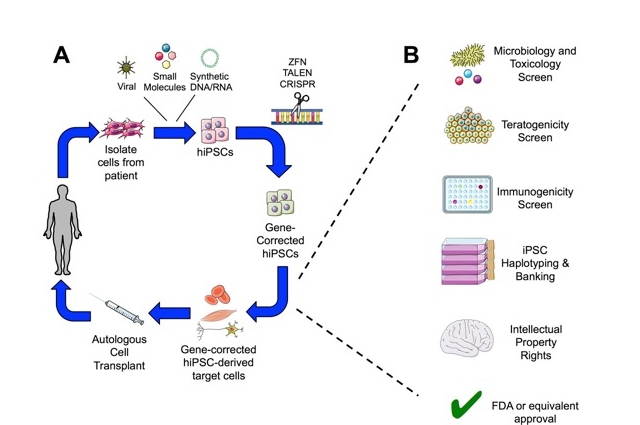
Figure from Angelos & Kaufman, 2015
The Kaufman laboratory uses human pluripotent stem cells to understand the development of blood cells. We primarily use human induced pluripotent stem cells (iPSCs) as a resource to produce blood and immune cells for new clinical applications for treatment of relapsed/refractory cancers — both hematologic malignancies and solid tumors. We have pioneered strategies to use iPSCs as a platform to produce engineered natural killer (NK) cells with improved anti-tumor activities. Engineered iPSC-derived NK cells are now in clinical trials using technology developed in our lab. These iPSC-NK cells have demonstrated both safety and efficacy.
We also derive and engineer monocytes and macrophage from human iPSCs that can be used for tissue repair and regeneration, as well as cancer therapies. We have also started work for new strategies on directly engineering immune cells directly within patients using either targeted lipid nanoparticles or virus-like particles.
Dr. Kaufman also provides clinical care for patients with hematological malignancies, with special interest in blood and marrow transplantation (BMT), cellular therapies, and immune therapies.
The main focus in our research group is to use human pluripotent stem cells as a platform to derive improved cell-based therapies. One key goal since human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) were first isolated or produced has been to use these pluripotent stem cells as a source for in vitro-derived hematopoietic cells that can be used to treat patients with novel cell-based therapies. Studies in the Kaufman Lab were the first to produce blood cells from hESCs (Kaufman et al. PNAS, 2001). While we spent considerable time trying to produce putative hematopoietic stem cells (HSCs) capable of long-term, multi-lineage engraftment, we have largely shifted to study development and engineering of immune cells with improved anti-tumor activity.
We have pioneered the use of human induced pluripotent stem cells (iPSCs) that can be engineered on the stem cell-level can routinely produce iPSC-derived natural killer (NK) cells that uniformly express genetic modifications. This technique allows production of iPSC-derived NK cells that express proteins such as chimeric antigen receptors (CARs) and cytokine signaling receptors or with target genes disrupted ("knocked-out").
Multiple publications from our group highlight the power of these approaches. First, we demonstrated iPSC-NK cells that express NK cell-optimized CARs were shown to mediate strong antigen-specific NK cell activation. These CAR-NK cells demonstrated increased anti-tumor killing against both solid tumors and hematologic malignancies (Li et al. Cell Stem Cells, 2018). A second series of studies showed that expression of a non-cleavable CD16 Fc-receptor in iPSC-NK cells mediated increased antibody-dependent cellular cytotoxicity against a range of target tumors. The combination of these engineered NK cells with monoclonal antibodies led to increased killing of tumors that are more resistant to NK cell killing (Zhu et al. Blood, 2020). Additionally, we demonstrated that deletion of internal genes can also improve the function of iPSC-derived NK cells. Knockout of the gene CISH, a negative regulator of cytokine signaling, in iPSC-NK cells was shown to increase NK cell expansion and killing of tumors. This approach demonstrated that disruption of genes involved in NK cell signaling and metabolism can generate NK cells with increased anti-tumor activity (Zhu et al. Cell Stem Cells, 2020). More recently, we have engineered iPSC-derived NK cells to be resistant to the immune inhibitory effects of TGFb in the tumor microenvironment (presented: Thangaraj, et al., Society for Immunotherapy of Cancer (SITC) annual meeting, 2023). We have also produced iPSC-NK cells that are resistant to drugs such as venetoclax or glucocorticoids to improve their anti-tumor activity (presented: Goldenson, et al., American Society for Hematology Annual meeting, 2023.
Several of these approaches have been licensed from our group and are now in clinical trials against challenging, refractory malignancies. These initial trials have demonstrated both safety and efficacy. Ongoing efforts are testing new CAR strategies and other genetic modifications to build newer, improved iPSC-derived NK cells.
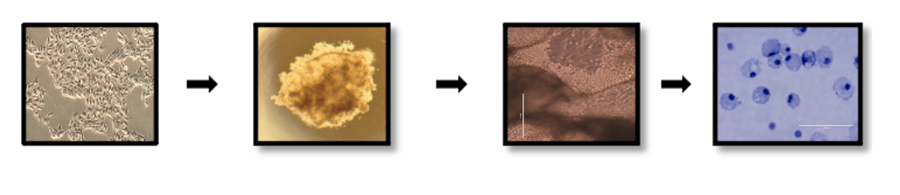
Studies from our group also demonstrate that it is very efficient to derive monocytes and macrophages from human pluripotent stem cells. We can also polarize these iPSC-derived macrophages to M1 and M2-type macrophages. M1 macrophages have pro-inflammatory, anti-tumor activity, whereas M2 macrophages have anti-inflammatory activity that can help mediate tissue repair and regeneration. We have used our approach to develop macrophage-specific CARs to derive iPSC-CAR-macrophages to better target more resistant solid-tumor malignancies. We have also demonstrated iPSC-macrophages can mediate repair in a xenograft model of hepatic fibrosis (Pouyanfard, et. al., Stem Cells, 2021).
Instead of producing engineered immune cells in the lab outside of the body, what if we could directly engineer patient immune cells within their body to become potent cancer fighters? We have recently begun using two different approaches to efficiently and safely achieve this goal. First, in collaboration with Dr. Ester Kwon’s lab (UCSD Dept. of Bioengineering) we are using lipid nanoparticles (LNPs) that contain mRNAs for CARs or other agents to produce in vivo CAR-engineered immune cells. Second, we have developed a novel platform for virus-like particles (VLPs) that are not infectious and can be readily produced and engineered to target different immune cell populations in vivo to express CARs or other immune stimulating agents. We have taken a broad approach to separately in vivo target and engineer either LNPs or VLPs specifically to either T cells, NK cells or macrophages. Our initial studies demonstrate successful in vivo engineering using these approaches in a humanized immunodeficient mouse model using human ovarian cancer cells (presented: Beltran-Garcia, et al., American Society for Hematology Annual meeting, 2023).
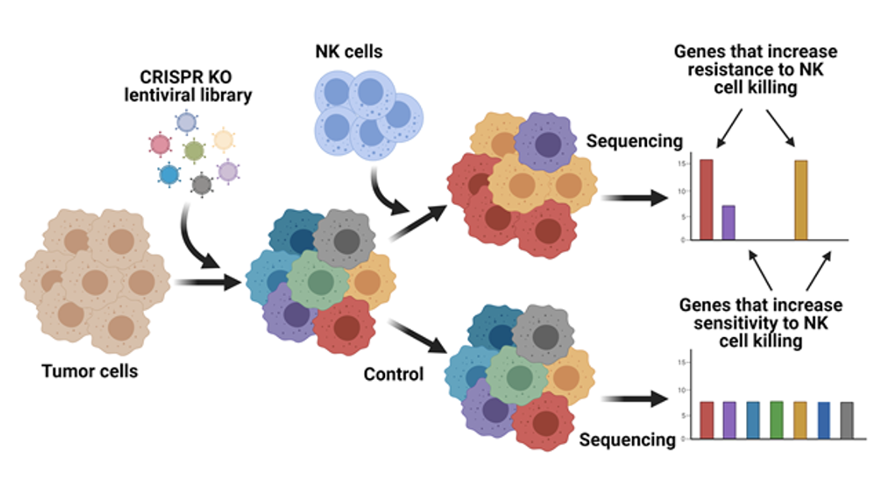
Inside a solid tumor the selective pressure pushes tumor cells to develop resistance mechanisms to escape NK cell-mediated killing. With the development of genome editing using CRISPR/Cas9 it is possible to identify new genes that are involved in tumor cell proliferation, drug resistance and metastasis of cancer cells. CRISPR libraries can be applied to screenings with genome-wide loss- or gain-of-functions. Studies in our group utilized a novel strategy using a "two cell type" CRISPR/Cas9 screening to identify mechanisms of resistance or sensitivity that will mediate better targeting tumor cells by NK cells. We identified a panel of potential genes that are essential in tumor cells for survival under selective pressure of NK cells. We specifically characterized the role of CHMP2A (a member of the ESRTIII complex) that is expressed in diverse malignancies to regulate NK cells and other immune cells to mediate anti-tumor activity (Bernareggi, et.,al., Nature Communications, 2022). Further tests of this strategy will allow us to identify other targets and to test pharmacological and genetic strategies to up or down-regulate molecular targets on tumor cells that could be translated into clinical trials to improve anti-tumor immunotherapy.
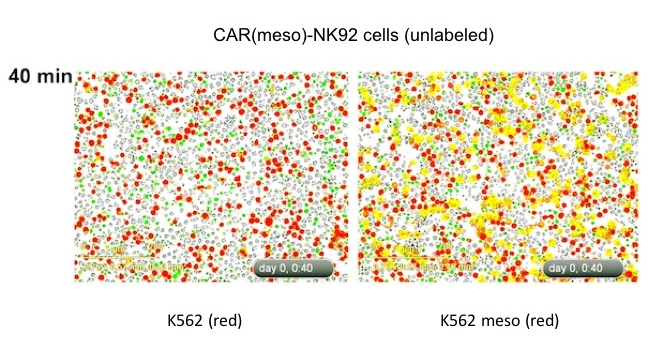
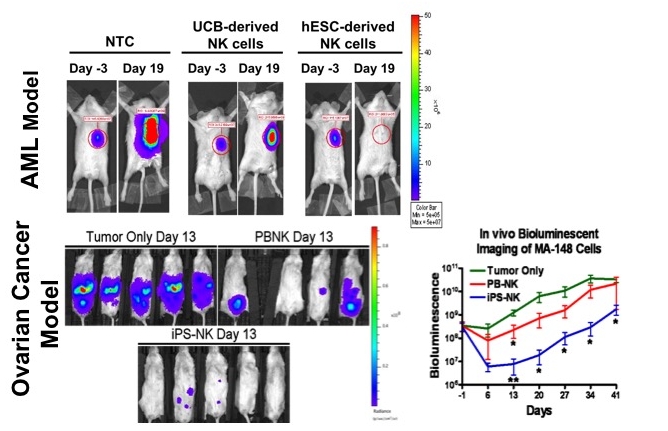
Figure from Woll, 2009 & Hermanson, 2016
Figure from Kaufman, 2018
Lineage-specific differentiation of osteogenic progenitors from pluripotent stem cells reveals the FGF1-RUNX2 association in neural crest-derived osteoprogenitors. Stem Cells. 2020 Sep;38(9):1107-1123.
Zhu H, Blum R, Bjordahl R, Gaidarova S, Rogers P, Lee TT, Abujarour R, Bonello GB, Wu J, Tsai PF, Miller JS, Walcheck B, Valamehr B, Kaufman DS. (2020). Pluripotent stem cell-derived NK cells with high-affinity non-cleavable CD16a mediate improved anti-tumor activity. Blood. 135(6):399-410.
Zhu H, Blum R, Bernareggi D, Ask EH, Wu Z, Hoel HJ, Meng Z, Wu C, Guan KL, Malmberg KJ, Kaufman DS. (2020). Metabolic Reprograming via Deletion of CISH in Human iPSC-Derived NK Cells Promotes In Vivo Persistence and Enhances Anti-tumor Activity. Cell Stem Cell. 27(2):224-237.
Bernareggi D, Pouyanfard S, Kaufman DS. (2019). Development of innate immune cells from human pluripotent stem cells. Exp Hematol. 71:13-23.
Li Y, Hermanson DL, Moriarity BS, Kaufman DS. (2018). Human iPSC-derived Natural Killer Cells Engineered with Chimeric Antigen Receptors Enhance Anti-Tumor Activity. Cell Stem Cell. 23(2), 181-192.
Zhu H, Lai YS, Li Y, Blum R, Kaufman DS. (2018). Concise Review: Human Pluripotent Stem Cells to Produce Cell-based Cancer Immunotherapy. Stem Cells. 36(2):134-145.
Angelos, M.G., Ruh, P.N., Webber, B.R., Blum, R.H., Ryan, C.D., Bendzick, L., Shim, S., Yingst, A.M., Tufa, D.M., Verneris, M.R., et al. (2017). Aryl hydrocarbon receptor inhibition promotes hematolymphoid development from human pluripotent stem cells. Blood 129, 3428-3439.
Hermanson, D.L., Bendzick, L., Pribyl, L., McCullar, V., Vogel, R.I., Miller, J.S., Geller, M.A., and Kaufman, D.S. (2016). Induced Pluripotent Stem Cell-Derived Natural Killer Cells for Treatment of Ovarian Cancer. Stem Cells 34, 93-101.
Angelos, M.G., and Kaufman, D.S. (2015). Pluripotent stem cell applications for regenerative medicine. Curr Opin Organ Transplant 20, 663-670.
Ferrell, P.I., Xi, J., Ma, C., Adlakha, M., and Kaufman, D.S. (2015). The RUNX1 +24 enhancer and P1 promoter identify a unique subpopulation of hematopoietic progenitor cells derived from human pluripotent stem cells. Stem Cells 33, 1130-1141.
Hermanson, D.L., and Kaufman, D.S. (2015). Utilizing chimeric antigen receptors to direct natural killer cell activity. Frontiers in immunology 6, 195.
Jing, Y., Ni, Z., Wu, J., Higgins, L.A., Markowski, T.W., Kaufman, D.S., and Walcheck, B. (2015). Identification of an ADAM17 Cleavage Region in Human CD16 (FcγRIII) and the Engineering of a Non-Cleavable Version of the Receptor in NK Cells. PLoS ONE, e0121788.
Ni, Z., Knorr, D.A., Bendzick, L., Allred, J., and Kaufman, D.S. (2014). Expression of chimeric receptor CD4ζ by natural killer cells derived from human pluripotent stem cells improves in vitro activity but does not enhance suppression of HIV infection in vivo. Stem Cells 32, 1021-1031.
Knorr, D.A., Ni, Z., Hermanson, D., Hexum, M.K., Bendzick, L., Cooper, L.J.N., Lee, D.A., and Kaufman, D.S. (2013). Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Translational Medicine 2, 274-283.
Kaufman, D.S. (2009). Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells. Blood 114, 3513-3523.
Tian, X., Hexum, M.K., Penchev, V.R., Taylor, R.J., Shultz, L.D., and Kaufman, D.S. (2009). Bioluminescent imaging demonstrates that transplanted human embryonic stem cell-derived CD34(+) cells preferentially develop into endothelial cells. Stem cells 27, 2675-2685.
Woll, P.S., Grzywacz, B., Tian, X., Marcus, R.K., Knorr, D.A., Verneris, M.R., and Kaufman, D.S. (2009). Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood 113, 6094-6101
Tian, X., Woll, P.S., Morris, J.K., Linehan, J.L., and Kaufman, D.S. (2006). Hematopoietic Engraftment of Human Embryonic Stem Cell-Derived Cells Is Regulated by Recipient Innate Immunity. Stem Cells 24, 1370-1380.
Kaufman, D.S., Hanson, E.T., Lewis, R.L., Auerbach, R., and Thomson, J.A. (2001). Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A 98, 10716-10721.